Protecting Health
Biomedical Use of Horseshoe Crabs
If you’ve ever had a flu shot, know someone with a pace maker or joint replacement, or have given your pet a rabies vaccination, you owe a debt of gratitude to the horseshoe crab. Vaccines, injectable drugs, intravenous solutions, and implantable medical devices, both for humans and animals, are quality checked for safety using a test that comes from the blood of horseshoe crabs.
From the 1940s through the late 1970s, rabbits were used to screen products such as pharmaceuticals for the presence of pyrogenic substances like endotoxin. The discovery of the horseshoe crabs’ immunological system, including the amebocyte blood cell and the blood clotting factors contained within, changed the way products were tested for endotoxin.

The copper-based “blue
blood” of the horseshoe crab
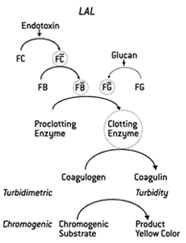
The cascade of enzymes
and proteins
The blood of the horseshoe crab is blue due to the copper-based oxygen carrying protein hemocyanin (see image). The amebocytes can be seen as the white pellet at the bottom of the blue liquid in the bottles.
The blood clotting system contains a cascade (see image) of three inactive enzymes and a clottable protein, coagulogen. In the presence of endotoxin, the first enzyme, Factor C is activated which activates Factor B. Factor B activates the clotting enzyme which cleaves coagulogen into coagulin. Coagulin molecules stick together to form a clot to protect the horseshoe crab from an exposure to Gram-negative bacteria or endotoxin. Frederik Bang and Jack Levin’s test uses the blood clotting system to form a gel clot in a test tube when endotoxoin is present in the test sample.
In 1977, the U.S. Food and Drug Administration allowed for the use of LAL as a replacement for the rabbit pyrogen test for detecting endotoxin in pharmaceuticals and medical devices (see An LAL Timeline for more). In the US it is still a requirement for a new drug to be tested in rabbits at least once before approval just to be sure there is not some inherent non-endotoxin pyrogen present.
This test now has worldwide acceptance either as LAL from the Limulus polyphemus or TAL from Tachypleus tridentatus.
Looking for more on the science? Visit our Research section.
How does horseshoe crab blood protect the public health?
Humans can become sick if exposed to bacterial endotoxin. It is especially dangerous if endotoxin enters our blood stream directly, such as from an injectable drug. Given this, drugs and medical devices that come in contact with our blood stream are tested to help assure they do not contain harmful levels of endotoxin.
Like other animals, the horseshoe crab has an immune and blood coagulation system that protects it against infection. Inside the horseshoe crab’s blood cell (called the amebocyte) are the protiens of its blood clotting system. These proteins are released in response to the presence of unwanted organisms like Gram negative bacteria and cause its blood to clot around the injury and bacteria, protecting the animal from further harm.
Endotoxin Testing: LAL and TAL
Research on horseshoe crabs showed that their blood is very sensitive to endotoxin, which is a component of Gram-negative bacteria like E. coli. In the 1960s (see timeline), Frederik Bang and Jack Levin developed a test from Limulus polyphemus blood that detected the presence of endotoxin. This test, based on the fact that the blood of the horseshoe crab gels or clots when it comes in contact with endotoxin, was called the Limulus amebocyte lysate (LAL)test and was commercialized in the United States in the 1970s. In Asia, there is a similar test called TAL which takes its name from an Asian species of crab, Tachypleus tridentatus.
The LAL and TAL test methods have advanced since the early days, all with the purpose of helping to make injectable drugs, vaccines, and medical devices safer for humans and animals. In addition to the use of their blood for an endotoxin test such as the gel clot test, the horseshoe crab’s DNA has been used to develop a recombinant test method for endotoxin. Even as alternatives are being developed that will retire or reduce the use of horseshoe crab blood, we will always be indebted to the horseshoe crab’s contribution to our health.
In addition, a version of the LAL test has been clear by FDA as a test to aid in the diagnosis of invasive fungal infections. This test takes advantage of the fact that the horseshoe crabs blood clotting system responds to (1→3)-β-glucans as well as to endotoxins. (1→3)-β-glucans are a cell wall component of a wide range of fungi. A glucan test can indicate the presence (or absence) of a fungal infection within a couple of hours of drawing a patient’s blood sample so that appropriate treatment can begin promptly. Conventional methods of diagnosis can take days of even weeks to give a definitive result.