Protecting Health
Biomedical Horseshoe Crab Harvest Mortality
LAL Harvest and Mortality
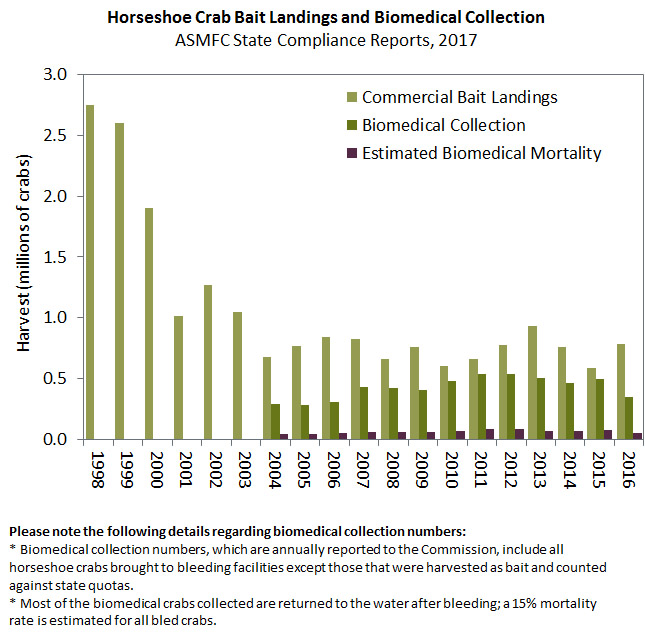
From the 1850s to the 1920s, between 1.5 and two million horseshoe crabs were harvested annually for fertilizer and livestock feed. Harvest dropped throughout the 1950s and ceased in the 1960s. Between 1970 and 1990, reported commercial harvest ranged from less than 20,000 pounds to greater than two million pounds annually. Since the mid- to late 1990s, commercial harvest has been sold primarily as bait for the American eel and whelk pot fisheries. Increased need for bait in the whelk fishery likely caused an increase in horseshoe crab harvest in the 1990s, with a peak of nearly six million pounds in 1997. Reported coastwide bait landings in 2016 remained well below the coastwide quota at 787,223 crabs.
Horseshoe crabs are also collected by the biomedical industry to support the production of LAL, or Limulus amoebocyte lysate, a clotting agent that aids in the detection of human pathogens in patients, drugs, and intravenous devices. No other procedure has achieved the same accuracy as the LAL test. Blood from the horseshoe crab is obtained by collecting adults and extracting a portion of their blood. Most crabs collected and bled by the biomedical industry are, as required by the FMP, released alive to the water from where they were collected; however, a portion of these crabs die from the procedure. Crabs harvested for bait are sometimes bled prior to being processed and sold by the bait industry; these crabs are counted against the bait quota. Since 2004, when reporting began, biomedical use has increased but has been fairly stable in recent years with an estimated 426,195 crabs brought to biomedical facilities in 2016. The Horseshoe Crab Management Board continues to collaborate with the biomedical industry to find ways to incorporate biomedical data into a regional stock assessment.
Commercial & Recreational Fisheries
Sustainability and Social Responsibility in Suppliers
A reliable and sustainable supply of raw materials is critical for pharmaceutical and medical device manufacturers to provide their products to patients on time. Sustainability in a supplier’s ability to provide raw materials is linked to their social and environmental business practices. Companies that act responsibly with how they treat their employees, how their facilities affect the environment and how they use natural resources are the types of suppliers with whom pharmaceutical and medical device companies should do business. Many companies have global citizenship, environmental sustainability or similar policies and include them on their websites.
LAL/TAL endotoxin detection kits are critical materials for the pharmaceutical and medical device industries. Without a reliable and sustainable source of LAL/TAL kits, products, such as injectable pharmaceuticals, cannot be cleared and released for commercial sale. Understanding how the LAL/TAL manufacturers manage the natural resource horseshoe crab, can help companies choose vendors that act responsibly and can promote the sustainability of the horseshoe crab species.
Best Practices in the U.S. |
Associates of Cape Cod (Wholly owned subsidiary of Seikagaku Corporation, Japan) | Charles River Endosafe (division of Charles River Laboratories, USA) | Lonza Walkersville (division of Lonza Group, Switzerland) | FUJIFILM Wako Chemicals U.S.A. Corporation (division of FUJIFILM Japan) |
| Harvesting: Collection Method (biomedical permit required) |
hand harvest: 100% |
trawling: 20% hand harvest: 80% |
trawling: 100% |
trawling: 100% |
| Harvesting: Utilize crabs from Bait market? | Yes | No | No | No |
| Husbandry: Moist and temperature-controlled transport | yes:100% | yes:100% | yes:100% | yes:100% |
| Husbandry: Prescreening for injured crabs | yes:100% | yes:100% | yes:100% | yes:100% |
| Husbandry: Separating crabs to avoid re-bleeding |
yes:100% | yes:100% | yes:100% | yes:100% |
| Bleeding: Aseptic environment and sterile needle use | yes:100% | yes:100% | yes:100% | yes:100% |
| Release: Returns crabs to point of origin | yes:100% | yes:100% | yes:100% | yes:100% |
| Release: Biomedical crabs released within 24 hours | 100% in 24 hours |
100% in 48 hours |
100% in 48 hours |
100% in 48 hours |
| Release: Bait crabs returned to vendor | 100% in 24 hours |
N/A | N/A | N/A |
| Release: Crabs tagged prior to return | yes: 100% biomedical crabs are marked with non-toxic paint | yes: 50% | yes: 100% | yes: 50% |
| Regulations: Comply with all government harvesting and conservation requirements | yes:100% | yes:100% | yes:100% | yes:100% |
| Regulations: Comply with ASMFC Best Practices document | yes:100% | yes:100% | yes:100% | yes:100% |
| Disclosure: Company makes HSC use and mortality data public | no | no | no | no |
Best Practices in Asia |
Fuzhou Xinbei Biochemical Industrial | Xiamen Chinese Horseshoe Crab Reagent Manufactory | Zhanjiang A&C Biological (Division of Charles River Laboratories) | Zhanjiang Bokang Marine Biological Co. Ltd. |
| Harvesting: Collection Method | trawling: 100% |
trawling: 100% |
trawling: 100% |
trawling: 100% |
| Husbandry: Moist and temperature-controlled transport | yes: 40% | yes: 40% | yes: 40% | yes: 40% |
| Husbandry: Prescreening for injured crabs | no | no | no | no |
| Husbandry: Separating crabs to avoid re-bleeding | no | no | no | no |
| Bleeding: Aseptic environment and sterile needle use | yes:100% | yes:100% | yes:100% | yes:100% |
| Release: Returns crabs to point of origin | no, 100% mortality | no, 100% mortality | no, 100% mortality | no, 100% mortality |
| Release: Return within 24 hours | no, 100% mortality | no, 100% mortality | no, 100% mortality | no, 100% mortality |
| Release: Return to point of origin | no, 100% mortality | no, 100% mortality | no, 100% mortality | no, 100% mortality |
| Release: Crab tagging prior to return | no, 100% mortality | no, 100% mortality | no, 100% mortality | no, 100% mortality |
| Regulations: Comply with all government harvesting and conservation requirements | yes:100% | yes:100% | yes:100% | yes:100% |
| Disclosure: Company makes HSC use and mortality data public | no | no | no | no |
See our list of companies that purchase their endotoxin detection materials from sustainable and responsible manufacturers.